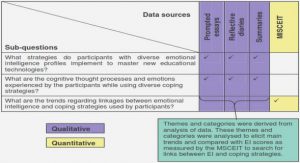
Get Complete Project Material File(s) Now! »
Atoms and photons in cavity
We investigate the interaction between two-level atoms and few photons confined in a very high Q cavity. The current setup we use was completed during the thesis of Sébastien Gleyzes [39]. The long lifetime of the field confined in the cavity during which we can measure the field about 800 times enables us to perform a feedback experiment. This chapter is devoted to the introduction of the experimental tools. We first show the experimental scheme, then present the two-level atoms, the high Q cavity, the theories describing their interactions and measurement of the field state.
In section I.1, we present the circular Rydberg states of Rubidium atoms, including their special properties as two-level systems, the preparation and detection methods. Besides these technical aspects, we also explain the theoretical description of these two-level systems. At the end, we discuss the principle of a Ramsey interferometer. In section I.2, we introduce the high Q cavity and the description of the photon field. The coupling of the field to its environment is also discussed. In section I.3, we present the theory describing the interaction between the atoms and photons. Depending on their relative frequencies, the two systems may interact dispersively or resonantly. In section I.4, we discuss the quantum nondemolition (QND) measurement of photon numbers. We start by recalling the postulates on measurement in quantum mechanics, then explain the principle of the QND measurement. Afterwards, we explain the methods for field state reconstruction and measurement of phase shift per photon.
Experimental setup
The experimental scheme is displayed in Fig. I.1. Let us follow the long blue arrow showing the direction of the atomic beam to briefly present the main components of the setup.
Oven
Rubidium atoms are stored in the oven. They are heated up, then diffuse out of it through a small hole. After the velocity selection process, only the atoms with a selected velocity participate in experiments.
Circularization box
The atoms are excited to the circular Rydberg states in B. This process involves several lasers, microwave and RF fields, etc. The small entrance hole on this box and the exit hole of the oven define the quasi unidimensional atomic beam.
Figure I.1. The experimental scheme. The long blue arrows shows the atomic beam. The torus-shaped elements represent circular atoms.
Ramsey zones and cavities
The two Ramsey zones R1, R2 and the high Q cavity C constitute the core part of the experiment. They are shielded from thermal and magnetic fields by a metal box. The box is cooled down to 0:8 K, by combining a Nitrogen cryostat, a 4He cryostat and a 3He refrigerator. The almost completely closed structure of the box suppresses the leakage of thermal photons into it, thus guarantees a well thermally equilibrated environment at 0:8 K for the cavity field. The two Ramsey zones are fed by the same microwave synthesizer through holes on one of their mirrors. The cavity is fed by another microwave synthesizer from one side. This design avoids damaging the mirror surface with a coupling hole and helps to obtain a high finesse cavity.
Detector
We apply an electric field between the two electrodes of D. The atom is then ionized, leaving a free electron focused by electric lens and detected by an electron multiplier.
Two-level atoms
Circular Rydberg atoms
The atom with an electron excited to energy levels with very high principal quantum numbers n is called a Rydberg atom. It is further designated as a “circular” Rydberg atom if that electron is excited to the level with maximal orbital and magnetic quantum numbers, i.e. l = m = n 1, because in this situation the electron has a circular orbit in the classical limit. Let us use jnci to denote such circular states. In our experiment we use the two circular levels j50ci and j51ci of 85Rb, which are also denoted as jgi and jei, respectively. Figure I.2 shows these levels and their energy difference. Their properties, preparation and detection will be described in the following.
Figure I.2. Energy levels of the circular Rydberg states used in our experiments.
Properties
Hydrogen-like energy levels
In the circular states jgi and jei, the valence electron is far away from the nucleus (the distance is much larger than the Bohr radius), the Rb atom thus has a hydrogen-like structure, in which the nucleus and all inner core electrons serve as a “heavier proton”. Within a very good approximation, the energy levels of this system can be written as Ry En = n2 ; where Ry is the slightly modified Rydberg constant that takes into account the mass of the “heavier proton” [39].
Under the condition n 1, the transition frequency between two neighboring circular levels reads
Ry Ry !n = 1 1 ’ 2 : ~ (n 1)2 n2 ~ n3
For states with n 50, the transition frequency 51:099 GHz falls in the microwave regime, a regime where there exist high quality field resonators, facilitating the search for high Q cavities.
Large electric dipole
The jnci ! j(n 1)ci transition is +-circularly polarized. given by [40, p. 254] d = n2 jqja0 ; n p2
Its dipole matrix element is (I.1) with q being the electron charge and a0 = 0:53 Å the Bohr radius.
Note that this value is proportional to n2, since the radius of a Rydberg atom is propor-tional to n2 in a hydrogen-like approximation. For the transition j50ci ! j51ci, we have d51 = 1776jqja0, which is a large number on the atomic scale.
Long lifetime
Among all states with a given principal quantum number, the circular states have the longest radiative lifetime [41]. In fact, the circular Rydberg state can only decay to the first lower circular level by spontaneous emission, i.e. jnci ! j (n 1) ci, according to the selection rules. This transition occurs at a frequency !n / n 3, with dn / n2. So the lifetime of the circular level reads: Tat = at1 = / n5: (I.2) 3 « 0 ~c
We see that Tat is proportional to n5. Particularly, the circular levels j50ci and j51ci have lifetimes 28:5 ms and 31:5 ms, respectively.
Quadratic Stark effect
Circular Rydberg atoms have long lifetimes and strong coupling to microwave field, which enable them to be good candidates as two-level systems. However, they are also very fragile in the sense that they can mix with other levels which have the same principal quantum number n, the so-called “elliptical” levels, because all these levels are degenerate in the absence of external electric or magnetic fields. These elliptical and circular states form the hydrogenic multiplicity of principal quantum number n.
The application of an external electric field F lifts this degeneracy. In this situation, l is no longer a good quantum number, since F breaks the spherical symmetry. But m, related to the symmetry around the z axis, remains as a good quantum number. The new eigenstates of the Hamiltonian can be denoted as jn; n1; mi, with n1 being the parabolic quantum number and satisfying 0 n1 (n j mj 1). Circular states have n1 = 0. It can be shown [42] that with a development up to the second order in F , the energy levels in the multiplicity of n can be expressed as E = E(0) + E(1) + E(2), with the three terms given by
E(0) = 1 (I.3)
E(1) = 3 knF (I.4)
E(2) = 1 17n2 3k2 9m2 + 19 (I.5)
with k = 2n1 n + jmj + 1. The energy and field amplitude are expressed in the atomic units. States jei and jgi together with the related elliptical levels are shown in Fig. I.3.
Figure I.3. Energy levels of the multiplicity of n = 50 and n = 51 in the presence of an external electric field.
Note that the linear Stark effect lifts the degeneracy between the circular level (m = n 1) and the levels with m = n 2 in the same multiplicity by 100 MHz=(V=cm). Thus in the presence of even a relatively weak electric field, the transitions starting from j(n 1)ci to jnci ( m = +1) or to elliptical levels jn; n1; m = n 2i ( m = 0) can be well distinguished. The transition as depicted in Fig. I.3 has a frequency very close to that between the two circular levels. However, the matrix element of its electric dipole jdj = 27jq ja0 is much smaller than that of the circular levels jdj + = 1776jqja0, making it negligible. To summarize, by applying an external electric field, we obtain a system with two levels well separated from other levels. This electric field, known as a guiding electric field, should have non zero values along the whole path of the circular Rydberg atoms in order to avoid mixing between the circular and elliptical levels. But its amplitude and direction can vary smoothly.
Also note that the linear Stark effect is zero for a circular level, but a quadratic term remains and shifts the frequency of jgi ! jei transition by 255 kHz=(V=cm)2. This effect is quite useful for tuning the atomic frequency in experiments. For instance, the atoms can be tuned to be on or off resonance with the cavity field by adjusting the static electric field between the cavity mirrors.
Preparation
The preparation of circular atoms is a complex process, in which we need to transfer a lot of energy and angular momentum to the atoms being initially in their ground state. The whole process, involving the participation of several lasers, RF field and varying electric field, etc., is shown in Fig. I.4. Since detailed explanation can be found in the thesis of Tristan Meunier [43], here we only outline the main procedures.
Circularization of Rubidium atoms
The excitation of atoms to the circular levels is performed in the presence of a magnetic field of 18 G. The atoms are first excited from the fundamental level 5S1=2; F = 3; mF = +3 to the j 52f; m = 2 i 780 nm, 776 nm and 1:26 m.
Rydberg level by three lasers at the wavelengths
The laser at 780 nm also plays a role of optical pumping, which brings atoms at other sub-levels of 5S1=2; F = 3 to 5S1=2; F = 3; mF = +3 . This laser is also pulsed with a typical duration 2 s , which defines a time origin for all following pulses.
The laser excitation process is performed in the absence of electric field. Then the electric field is gradually switched on and lifts the degeneracy of the multiplicity of n = 52. Atoms are then transferred to the level jn = 52; n1 = 1; m = 2i. The electric field is then swept and a RF field is turned on at the same time, transferring atoms to the circular level j52ci through a rapid adiabatic passage during which 49 photons at 255 MHz are absorbed. In this process, the degeneracy of + and transitions are lifted by the magnetic field at 18 G. This field, however, needs to be shielded in order not to perturb, by the Zeeman effect, the circular levels going into the cavity. In the setup, we use a cylinder container made of superconducting Niobium to shield the magnetic field. It is in this container, also called the circularization box, that the circular levels are prepared.
Nevertheless, the prepared circular states are not pure, because some atoms may end up in one of the elliptical states during the adiabatic passage. So after the preparation of state j52ci, a purification microwave is switched on, which brings atoms to j51ci by one-photon transition at 48:2 GHz, or j50ci by two-photon transition at 49:6 GHz. Eventually, the atoms staying on the levels of n = 52, no matter circular or elliptical, are ionized by a strong electric field at the exit of the circularization box. At the end, we can obtain pure circular levels jei or jgi. In all experiments discussed in this manuscript, the atoms are prepared in the state jgi in this process.
Two-level atoms
Controlling atomic position and velocity
Controlling the transverse profile of the atomic beam is necessary in order to reduce the effect of inhomogeneous static electric field and to let the atoms pass through the center of the cavity, such that strongest coupling to the cavity field is achieved. A quasi unidimensional atomic beam is selected by the exit hole of the oven and the entrance hole of the circularization box. The Rubidium atoms contained in the oven are heated up to about 185 C. They diffuse out of the oven at their thermal velocities through a 0:7 mm hole and need to pass through another 0:7 mm hole at the entrance of the circularization box before entering the cavity. The two holes, separated by 550 mm, thus select an atomic beam with a transverse diameter of about 0:7 mm.
Thermal velocities of the atoms satisfy a Maxwell-Boltzmann distribution, which has a width of about 270 m=s. In experiments, we need to know the position of atoms at a given time, in other words, the velocity of atoms should be well selected and controlled. This is done by two techniques: the first is velocity selection using Doppler effect and the second is flight time selection. The principle of the first technique is shown in Fig. I.5.
Figure I.5. Velocity selection using Doppler effect. (a) Directions of the de-pumper and re-pumper lasers relative to the atomic beam. (b) Hyperfine structure of the atomic levels. The de-pumper laser depopulates the level jF = 3i by pumping atoms to jF = 2i via jF 0 = 3i. (c) The re-pumper laser brings atoms with velocity v = ! rep=2 cos from jF = 2i to jF = 3i via jF 0 = 3i. Another velocity class v0 = !0 rep=2 cos is also selected via jF 0 = 2i.
The de-pumper laser, resonant with the transition 5S1=2; F = 3 ! 5P3=2;F0 = 3 , is always on. It depopulates the level 5S ;F =3 by pumping all atoms to the lower 1=2 hyperfine level laser, at an angle 63 to the atomic beam, 5S1=2; F = 2 . The re-pumper ! is detuned by with respect to the transition 5S ;F =2 5P ; F 0 = 3 . Due to ! 1=2 3=2
Doppler effect, only the atoms with velocity v = ! =2 cos see a laser frequency shift rep which compensates !. They are thus pumped to the level jF = 3i via jF 0 = 3i. Beside this velocity class, another class v0 = !0 rep=2 cos can also be selected via F 0 = 2. The double peaked curve in Fig. I.6 shows the selected velocity classes.
The full width at half maximum (FWHM) of the velocity distribution after selection by the Doppler effect is about v = 30 m=s. It leads to a longitudinal dispersion of about 3 cm at the position of the detector, which is much larger than the 6 mm hole on the detector. So we use flight time selection to further reduce the velocity dispersion. As mentioned before, the excitation laser at 780 nm is pulsed, with a typical duration of 2 s. Since the re-pumper laser is separated from this laser by about 340 mm, we can also make the re-pumper laser pulsed, with its timing adjusted such that only atoms with the selected velocity can arrive in B when the excitation process starts. The velocity distribution thus obtained around 250 m=s is shown in the inset of Fig. I.6. As an example, using a 4 s pulse for re-pumper laser, we can obtain a velocity class of (250 2) m=s, resulting in a longitudinal length of about 4 mm, which is smaller than the hole on the detector.
Table of contents :
Introduction
I Atoms and photons in cavity
I.1 Two-level atoms
I.1.1 Circular Rydberg atoms
I.1.1.a Properties
I.1.1.b Preparation
I.1.1.c Detection
I.1.2 Theory of two-level systems
I.1.2.a Atomic pseudo spin and Bloch sphere
I.1.2.b Manipulation of atomic states
I.1.2.c Ramsey interferometer
I.2 Photons in cavity
I.2.1 The high finesse Fabry-Pérot cavity
I.2.2 Theoretical description
I.2.2.a A quantized field
I.2.2.b Coupling to the environment
I.3 Coupling the two systems
I.3.1 Resonant regime
I.3.2 Dispersive regime
I.3.3 Rapid adiabatic passage
I.4 Measurement of field state
I.4.1 General measurement theory
I.4.2 Quantum nondemolition measurement of photon numbers
I.4.2.a Measurement principle
I.4.2.b Modification of photon number distribution
I.4.2.c Reconstruction of photon number distributions
I.4.2.d Phase shifts in photon number states
II Quantum feedback: state estimation
II.1 General principle
II.1.1 From classical to quantum feedback
II.1.2 Principle of the quantum feedback experiment
II.2 Sensor samples
II.3 Actuator samples
II.3.1 Sample with 1 atom
II.3.1.a Ideal resonant interaction
II.3.1.b Imperfect Rabi oscillations
II.3.1.c Rabi oscillations in the Fock states
II.3.1.d Mixing of the Kraus operators
II.3.2 Sample with 2 atoms
II.3.2.a Ideal resonant interaction
II.3.2.b Vacuum Rabi oscillations of two atoms
II.3.3 Quantum maps
II.4 State estimation
III Quantum feedback: algorithms and optimizations
III.1 Complete timing of the feedback experiment
III.2 Quantum state estimator
III.3 Controller
III.3.1 Measure of distance
III.3.2 Decision making
III.4 Simulations and optimization of parameters
III.4.1 Parameters for the simulations
III.4.2 Results of the simulations
III.4.3 Optimizations
III.4.3.a The partition of samples
III.4.3.b Distance functions
III.4.3.c The interaction time for actuators
IV Experimental implementation
IV.1 Control systems
IV.1.1 Organization of the control systems
IV.1.2 Main components of the Active system
IV.2 Sequence
IV.2.1 Experimental events for one sample
IV.2.2 The feedback sequence
IV.2.3 The measurement sequence
IV.3 Results
IV.3.1 Individual trajectories
IV.3.2 Photon number distributions
IV.3.2.a Stationary regime
IV.3.2.b Termination by a threshold on fidelity
IV.3.3 Behavior of the controller
IV.3.3.a Sample types in the stationary regime
IV.3.3.b Sample types dependent on the mean photon number
Conclusion
A Two-atom Rabi oscillations
B Simulation procedure
Bibliography