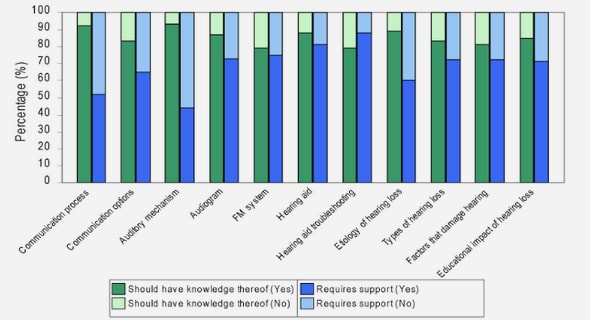
Get Complete Project Material File(s) Now! »
Why are mountains species rich ?
Multiple hypotheses have been proposed to explain the exceptional diversity of mountain regions. These explanations differ in the processes they rely on, but also in the diversity components and phenomena they explain. In particular, there are explanations for higher richness or endemism of mountains compared to surrounding flat regions, but also explanations for higher endemism and faster diversification specifically in high elevation areas (see Box 1 for definitions of mountains and high elevation areas). The majority of presented explanations are not mutually exclusive, and dominant ones are likely to differ in different mountain systems.
The most simplistic explanations of why are mountains species rich do not account for evolutionary processes and explain richness of the mountains systems solely by ecological mechanisms. One of such explanations is that a unit of map area in rugged regions contains more surface inhabitable by biota than a unit of map area in flat regions, leading to higher richness simply due to more physical space available (Fig. 1A). This explanation was originally developed for underwater systems (Kostylev et al., 2005), and up to my knowledge it has never been seriously considered in terrestrial ecosystems. However, this simple geometric property of rugged landscapes may be relevant for organisms that are limited by resources distributed according to surface rather than according to map area. An example of such organisms may be plants in rocky habitats, limited rather by availability of rooting opportunities in rock crevices than by light. Another purely ecological explanation is that mountains are rich because they host higher diversity of ecological conditions than flat areas, allowing the ecological assembly of more species specialized for different niches (sensu Chesson, 2000; Fig. 1B).
Both ecological mechanisms can explain higher richness of mountain regions, but they fail to tackle another notable pattern of mountain biodiversity, which is high endemism (Steinbauer et al., 2016). It suggests that in most situations, they must be coupled with other explanations accounting for evolutionary processes capable of producing endemism. For example, the higher richness of habitats may allow ecological assembly of more species, but on a temporal scale it may also trigger adaptive diversification to specific habitats (reviewed below), generating endemism. The main aim of this thesis is studying diversification, rather than ecological assembly, but the purely ecological explanations why are mountains species rich are an important null models to consider when exploring more complex explanations. Moreover, there are specific mountain regions with extremely low endemism, where the biota indeed resulted mostly from ecological assembly of immigrant species, for example the flora of arctic mountain regions emerged almost completely from post-glacial recolonization (Brochmann et al., 2003) and little in-situ diversification could take place after the glaciation.
Higher endemism in mountains may be connected to the fact that some mountain regions are more climatically stable than surrounding lowlands and may thus host paleoendemic species that got extinct anywhere else. Climatic stability in mountain regions in the past is related to two factors: first, rugged terrains provide species the opportunity to migrate across the altitudinal gradient to buffer temperature changes with little migration in space (Fig. 1C; Feng et al., 2016); second, during the Pleistocene glacial periods a very important limiting factor for vegetation was humidity, and in many regions, mountains constituted humid refugia that allowed a higher survival rate of species than in dry lowland landscapes (Fig. 1D; Birks and Willis, 2008; Schmitt, 2007). On the other hand, certain mountain ranges or their parts may have been climatically less stable than surrounding lowlands – an extreme example are the mountain ranges that were severely glaciated during the Pleistocene ice ages. Mountains may thus create a mosaic of regions that were more climatically stable during the Pleistocene than surrounding lowlands (i.e. refugia) and regions where almost all biota was eradicated in some phases of Pleistocene glacial cycles (Feng et al., 2016; Tribsch and Schönswetter, 2003). Where the stability explanation of high endemism is the case, a region should be a species museum (sensu Stenseth, 1984) with diversity in a large part consisting of rare ancient lineages.
The museum explanation may apply to some regions, but most mountain regions (including those that constitute global biodiversity hotspots) host many neoendemic species and lineages that diversified in recent times (Hughes and Atchinson, 2015; Pouchon et al., 2018; Xing and Ree, 2017), suggesting that such regions are species cradles rather than museums (sensu Stenseth, 1984). In fact, some radiations in tropical mountains, e.g. Lupinus in the Andes, are considered the fastest documented examples of diversification in the world (Hughes and Eastwood, 2006). There are several explanations of faster diversification in mountain regions, having specific ecological, geographical and temporal consequences.
The first explanation is linked with the aforementioned explanation related to high diversity of environmental conditions, adding a dynamic evolutionary component. That is, diversification can be stimulated by the presence of many different habitats on a relatively small area, fostering adaptive differentiation of populations and ecological sympatric or parapatric speciation (Fig. 1E). If this mechanism is the predominant one, speciation events would take place across the major ecological gradients in the mountains, such as the elevational gradient (associated with gradients of temperature and precipitation), and for plants also across different bedrocks (calcareous vs siliceous bedrocks) or soil depths (deep soils vs bare rock). Indeed, it has already been documented that speciation events may be in some cases associated with elevational niche change (Hughes and Atchinson, 2015; Luebert and Weigend, 2014; Merckx et al., 2015; Pouchon et al., 2018) or with change of bedrock preference (Boucher et al., 2016; Moore and Kadereit, 2013; Pachschwöll et al., 2015).
Another potential explanation is linked to insularity of mountains. Mountains can be considered as insular systems of specific habitats (“Sky Islands” sensu Heald, 1967), thus creating the opportunity for geographic isolation of populations and allopatric speciation (Fig. 1F). This mechanism may be further fostered by changing connectivity of habitats due to climatic changes, via a speciation pump effect sensu Haffer (1967). An important question concerning this explanation is at which spatial scale is the isolation relevant for allopatric speciation, given that mountains present insularity at different scales, from major mountain ranges of thousands to millions of square kilometers, to individual summits of massifs within the mountain ranges, or at even smaller scale, to patches of specific habitats such as rock crevices for plants. The expected pattern of biodiversity if the allopatric speciation predominates is that sister mountain lineages would present non-overlapping geographic distributions. For example, Boucher et al. (2016) explored the frequency of ecological vs allopatric speciation events in mountain Primulaceae in Europe, showing that allopatric speciation is indeed the dominant process. Importantly, different habitats present in the mountains may also exhibit different levels of insularity. For example high elevation habitats are typically more insular than lower elevation stands (although this might be the opposite in certain regions and geographic scales, such as in uplands divided by deep canyons in eastern Africa), and the more insular habitats should thus have higher rates of species diversification than the ones that are more connected.
There are also other explanation of diversification in mountains, predicting that diversification should be faster specifically in high elevation areas. Interestingly, these explanations typically do not predict that high elevation areas would be more rich in species, which is consistent with observed patterns – the species richness is typically declining with elevation or is eventually hump shaped due to mid-domain effects (Lomolino, 2001; Quintero and Jetz, 2018; Rahbek, 1995), while proportion of endemism and evolutionarily young species grows with elevation (Aeschimann et al., 2011; Quintero and Jetz, 2018; Steinbauer et al., 2016). But in turn, high endemism in high elevation areas, despite lower total richness, may be an explanation for higher richness of whole mountain regions containing such high elevation areas.
One of such high-elevation-specific explanations assumes that cold high elevation habitats are relatively younger than warm low elevation habitats, and the fast diversifications of high-elevation biota is triggered by the opportunity to colonize vacant ecological space (Fig. 1H; Hughes and Eastwood, 2006; Stroud and Losos, 2016). Such radiations would present particular evolutionary signatures. Their beginning should be coincident with orogenetic processes or with the appearance of key innovations allowing the colonization of high altitude habitats. Due to this, the diversity proportions in high and low elevation habitats should be out of equilibrium that is to be reached after saturation of diversification in high elevation habitats, and stabilize with carrying capacities not necessarily larger in high than in lower elevation habitats. There is evidence that diversification dynamics match with orogenetic processes in some cases, as it has been shown for the flora of Hengduan mountains (Xing and Ree, 2017), bellflowers in the Andes (Lagomarsino et al., 2016) or for Apollo butterflies in Eastern Asia (Condamine et al., 2018). Other studies have shown that diversification slows down after initial radiation triggered by the evolution of morphological adaptations to cope with harsh environmental conditions found at high elevations (Roquet et al., 2013). In all these examples, it can be assumed that the vacant ecological space mechanism does not work by itself, but in concert with others mechanisms such as those related to habitat diversity, or insularity of mountain regions.
There are other hypotheses aiming to explain higher diversification at high elevation that are either little tested, or can only apply to certain groups of organisms. For example, diversification in high elevations may be stimulated by higher UV irradiance causing faster molecular evolution (Fig. 1G; Cortés et al., 2018; Willis et al., 2009). Similarly, it was proposed that fast diversification in some mountain lineages may be linked to genome duplications, that may confer advantages for life in colder temperature and trigger speciation at the same time (Fig. 1J; Jordon-Thaden and Koch, 2008; Schönswetter et al., 2007; Theodoridis et al., 2013). The fast diversifications in insect-pollinated high elevation plants may also be linked to lower availability and mobility of pollinators in high elevation habitats (García-Camacho and Totland, 2009; Körner, 1999), leading to smaller connectivity of populations, which can favor their differentiation and finally lead to speciation (Fig. 1I).
Such specific mechanisms of diversification are fascinating subjects of study, but they do not provide a general answer to why high elevation areas are rich in endemics or why mountain biota diversifies faster, because they apply only to specific groups of organisms. The UV mechanism can only play an important role in organisms with germinal lineages cells long term exposed to outer environment, as are plants; polyploidisation is common in certain groups of vascular plants, but is very rare in others, and the later mechanism only applies to insect-pollinated plants. In contrast, high endemic richness and fast diversifications in high elevation areas are fairly general phenomena, taking place in biologically very disparate groups, as are vascular plants (Aeschimann et al., 2011; Hughes and Eastwood, 2006; Molina-Venegas et al., 2015; Pouchon et al., 2018), birds (Cai et al., 2018; Päckert et al., 2012; Quintero and Jetz, 2018), and insects (Chen et al., 2009; Merckx et al., 2015).
Explanations why mountains are rich in species (A-B) and in endemics (C-J). The explanations are further categorized based on whether they work with extinction (C-D) or speciation (E-J), and whether they are valid for mountain biotas as a whole (E-F) or only for high elevation biotas (G-J).
How to study diversification
The studies of diversification in mountains are often limited by availability of data, due to costs associated with gathering molecular phylogenetic datasets, and also due to limited accessibility of mountains complicating the collection of ecological or occurrence data in field. The choices of methodology, geographic and phylogenetic scale of the study are often driven by practical structure of available data, rather than theoretical assumptions about the study system. Such choices can however strongly influence the conclusions of evolutionary studies (Graham et al., 2018), and it is thus important to be aware of different methodological approaches, and of their strengths and weaknesses. In the literature, we can find two conceptual approaches regarding how to study diversification with data gathered from recent biota, each of them aiming on answering questions at different scales, i.e. with different amount of detail traded for potential of generalization.
The first approach, which could be called systematic or clade-oriented, consists in the study of diversification rates of a particular evolutionary lineage (Fig. 2A, e.g. genus Androsace in Roquet et al., 2013). A special case of systematic studies are phylogeographic studies, focusing on survival mechanisms within single species or diversification within single cladogenetic event, mapping for example divergence patterns between two newly emerging species at the level of individual populations (e.g. Kolář et al., 2016). The classical clade-oriented studies operating with multiple species aim on reconstructing their phylogenetic relationships using molecular data, optimally with a complete sampling of all species of the lineage. The phylogenetic trees are used to estimate diversification rates, their shifts and dynamics according to study-specific hypotheses. This can be done with process-based models considering diversification as a mechanistic branching process of the phylogenetic tree (reviewed in Morlon, 2014), as is Birth-Death model (BD; Nee et al., 1994), its modifications like State-dependent Speciation Extinction models (SSE; Maddison et al., 2007) or Bayesian Analysis of Macroevolutionary Mixtures (BAMM, Rabosky et al., 2014).
The advantage of these modeling approaches is that they can often directly stand for tested hypotheses, and as other process-based models, they can also be used for hind- or forecasting. The process-based diversification models can also in theory estimate separately speciation and extinction rates, although this ability in real studies has been questioned (Rabosky, 2010). A complementary modeling approach is the use of non-parametric estimators of lineage diversification as the gamma statistic (Pybus and Harvey, 2000), various designs of comparisons between sister species or lineages (Boucher et al., 2016), visual inspection of shapes of phylogenies (Vargas, 2003) or lineage-through-time plots (LTT; Kadereit et al., 2004). A great example of the systematic approach to diversification of European alpine flora is the study of the genus Phyteuma (Schneeweiss et al., 2013). In this study, the authors use molecular data to build a species level phylogeny and analyze it via mechanistic diversification model to conclude that the diversification process in genus Phyteuma is strongly driven by geographic range splits at shallow scales and by ploidy dynamics at deeper scales. The power of systematic studies lies in the highly resolved phylogenetic relationships and the possibility for mechanistic modeling of diversification dynamics of the selected lineage. On the other hand, as the focal lineage is selected non-randomly, these studies lack potential for generalization of findings beyond the focal lineage.
The other approach, which could be called the community-oriented, is to study diversification across a broader selection of evolutionary lineages, either all lineages present in the biota in certain region, or their stratified subsample (Fig. 2B, e.g. Molina-Venegas et al., 2015). This typically means that evolutionary information for each of the lineages is much less precise than in systematic studies, because constructing well resolved phylogenies across many lineages would be too costly and time-consuming. The emphasis on the community also means that phylogenetic information is gathered only for species that occur in the focal area, leading to paraphyletic sampling of lineages, because most areas are systems open to immigration and emigration. These two properties of the data mean that mechanistic diversification models or their nonparametric alternatives cannot be used here, because they require high quality phylogenetic data and either complete species sampling of the lineage or at least random subselection of species (Fitzjohn et al., 2009).
The analytical inference of community-oriented studies is thus limited to the exploration of spatial, ecological or temporal patterns of diversity indices that include the information about the evolutionary history, as are various phylogenetic diversity indices (reviewed in Tucker et al., 2016) such as phylogenetic diversity, mean terminal branch length (Feng et al., 2016) or more sophisticated measures like phylogenetic endemism (Mishler et al., 2014; Rosauer et al., 2009). A specific case are studies that test diversification hypotheses via patterns of diversity indices that do not explicitly include phylogenetic information, as are different measures of endemism (Pawłowski, 1970; Steinbauer et al., 2016; Tribsch and Schönswetter, 2003). To do this, they need to adopt the assumption that higher endemism indicates faster speciation or on the other hand slower extinction. An example of study using phylogenetic diversity indices to explore diversification patterns is Molina-Venegas et al. (2015). In this study the patterns of diversification across elevational, bedrock and geographic gradients in mountains of southern Spain are explored via phylogenetic alpha and beta diversity, to conclude that the strongest contributors to species diversification are geographic isolation and bedrock diversity, rather than elevational gradient. While the main power of the community-oriented approach lies in the study of a whole region and thus should allow generalizations, the main disadvantage lies in lower sensitivity due to phylogenetic data quality and also in the inability to efficiently separate different components generating diversity patterns, as are speciation, extinction or migration.
Table of contents :
GENERAL INTRODUCTION
Why are mountains species rich ?
How to study diversification
European mountains as a study system
Thesis aims and structure
References
CHAPTER 1
Abstract
Introduction
Methods
Results
Discussion
Acknowledgements
References
CHAPTER 2
Abstract
Introduction
Results
Discussion
Methods
Acknowledgements
References
CHAPTER 3
Abstract
Introduction
Methods
Results
Discussion
Future directions
Acknowledgements
References
GENERAL DISCUSSION
Past climatic dynamics and its influence on survival and diversification
Modes of diversification of the European mountain flora
Future directions
References
APPENDICES OF CHAPTER 1
APPENDICES OF CHAPTER 2
APPENDICES OF CHAPTER 3